About
I am a research assistant at the National Magnetic Resonance Research Center, working under Prof. Tolga Çukur. I received my M.Sc. in Electrical and Electronics Engineering at Bilkent University. I received my B.Sc. in Electrical Engineering at National University of Sciences and Technology.
My interests lie in the field of machine learning and computer vision. I employ deep learning techniques to develop innovative generative models specifically designed for the reconstruction of medical images.
Latest version of my curriculum vitae is available here
- Machine Learning
- Computer Vision
- Generative Models
MSc in Electrical and Electronics Engineering, 2021-2024
Bilkent University
BSc in Electrical Engineering, 2017-2021
National University of Sciences and Technology
Journal Publications
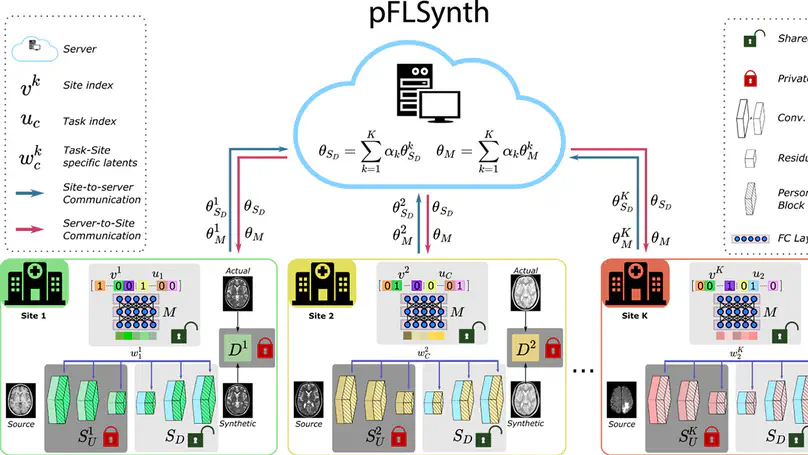
Curation of large, diverse MRI datasets via multi-institutional collaborations can help improve learning of generalizable synthesis models that reliably translate source- onto target-contrast images. To facilitate collaborations, federated learning (FL) adopts decentralized model training while mitigating privacy concerns by avoiding sharing of imaging data. However, conventional FL methods can be impaired by the inherent heterogeneity in the data distribution, with domain shifts evident within and across imaging sites. Here we introduce the first personalized FL method for MRI Synthesis (pFLSynth) that improves reliability against data heterogeneity via model specialization to individual sites and synthesis tasks (i.e., source-target contrasts). To do this, pFLSynth leverages an adversarial model equipped with novel personalization blocks that control the statistics of generated feature maps across the spatial/channel dimensions, given latent variables specific to sites and tasks. To further promote communication efficiency and site specialization, partial network aggregation is employed over later generator stages while earlier generator stages and the discriminator are trained locally. As such, pFLSynth enables multi-task training of multi-site synthesis models with high generalization performance across sites and tasks. Comprehensive experiments demonstrate the superior performance and reliability of pFLSynth in MRI synthesis against prior federated methods.
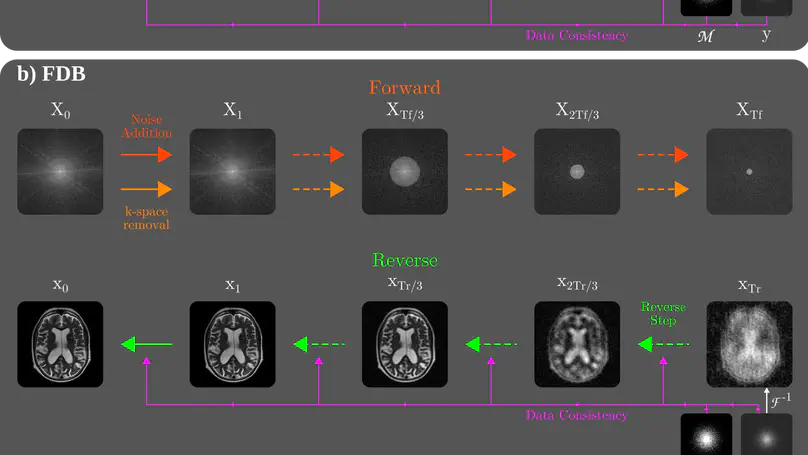
Recent years have witnessed a surge in deep generative models for accelerated MRI reconstruction. Diffusion priors in particular have gained traction with their superior representational fidelity and diversity. Instead of the target transformation from undersampled to fully-sampled data, common diffusion priors are trained to learn a multi-step transformation from Gaussian noise onto fully-sampled data. During inference, data-fidelity projections are injected in between reverse diffusion steps to reach a compromise solution within the span of both the diffusion prior and the imaging operator. Unfortunately, suboptimal solutions can arise as the normality assumption of the diffusion prior causes divergence between learned and target transformations. To address this limitation, here we introduce the first diffusion bridge for accelerated MRI reconstruction. The proposed Fourier-constrained diffusion bridge (FDB) leverages a generalized process to transform between undersampled and fully-sampled data via random noise addition and random frequency removal as degradation operators. Unlike common diffusion priors that use an asymptotic endpoint based on Gaussian noise, FDB captures a transformation between finite endpoints where the initial endpoint is based on moderate degradation of fully-sampled data. Demonstrations on brain MRI indicate that FDB outperforms state-of-the-art reconstruction methods including conventional diffusion priors.